Interventional laser surgery for oral potentially malignant disorders: a longitudinal patient cohort study
Keywords:medical, fibers, Time:27-04-2017Abstract
Oral squamous cell carcinoma (OSCC) is a lethal disease, with rising incidence. There were 6767 new OSCC cases and 2056 deaths in the UK in 2011. Cancers are preceded by oral potentially malignant disorders (PMDs), recognizable mucosal diseases harbouring increased SCC risk, offering clinicians a ‘therapeutic window’ to intervene. Contemporary practice remains unable to predict lesion behaviour or quantify malignant transformation risk. No clear management guidelines exist and it is unclear from the literature whether early diagnosis and intervention prevents cancer. Between 1996 and 2014, 773 laser treatments were performed on 590 PMD patients in Newcastle maxillofacial surgery departments. The efficacy of the intervention was examined by review of the clinicopathological details and clinical outcomes of the patients (mean follow-up 7.3 years). Histopathology required up-grading in 36.1% on examining excision specimens. Seventy-five percent of patients were disease-free, mostly younger patients with low-grade dysplasia; 9% exhibited persistent disease and were generally older with proliferative verrucous leukoplakia. Disease-free status was less likely for erythroleukoplakia (P = 0.022), ‘high-grade’ dysplasia (P < 0.0001), and with lichenoid inflammation (P = 0.028). Unexpected OSCC was identified in 12.0%, whilst 4.8% transformed to malignancy. Interventional laser surgery facilitates definitive diagnosis and treatment, allows early diagnosis of OSCC, identifies progressive disease, and defines outcome categories. Evidence is lacking that intervention halts carcinogenesis. Multicentre, prospective, randomized controlled trials are needed to confirm the efficacy of surgery.
Key words
oral potentially malignant disorders; interventional laser surgery; early diagnosis of cancer;dental laser tips
Oral squamous cell carcinoma (OSCC), probably the most common head and neck malignancy, remains lethal and deforming due to local invasion, orofacial destruction, metastasis to cervical lymph nodes, and blood–borne tumour dissemination.1 In 2011 there were 6767 new cases and 2056 deaths in the UK, whilst globally OSCC shows a rising incidence and a prognosis significantly compromised by advanced disease and late presentation.2
The ‘progression model’ for oral carcinogenesis proposes that, following irreversible genotypic mutation, phenotypic epithelial disorganization and dysmaturation changes occur, which, if allowed to progress, lead to invasive OSCC.3 Such features preceding cancer are identifiable microscopically and are collectively termed dysplasia.4 In clinical practice, assessment of the degree of dysplastic change is made following incision biopsy and classification into mild, moderate, or severe categories, according to the extent of atypical epithelium.5
It has been recognized for many years that a spectrum of distinct mucosal abnormalities, now termed potentially malignant disorders (PMDs) and which accompany dysplasia, may be identified clinically, albeit non-specifically, during oral examination. There is, therefore, an opportunity for early diagnosis and therapeutic intervention during this ‘oral pre-cancer window’.6 PMD encompasses primarily localized lesions such as leukoplakia, erythroplakia, and erythroleukoplakia, together with progressive multifocal disorders such as proliferative verrucous leukoplakia (PVL).7 ; 8
The identification of a PMD in a patient does not mean that malignant transformation is inevitable. Many lesions do not progress, whilst others resolve spontaneously, but it remains impossible in clinical practice to predict behaviour for individual cases. Nonetheless, patients with PMDs remain at increased risk of OSCC.9
‘Potential malignancy’ is difficult to define, so it is unsurprising that previous treatment interventions have been non-specific with ill-defined goals and end-points; the few clinical trials in the literature concentrate on medical therapies and are compromised by small numbers of patients and short study durations.10 No study has demonstrated long-term lesion resolution, reduction in disease incidence, or prevention of malignant transformation.11 Arduino et al. emphasized the need for long-term patient studies to improve understanding of the natural history of PMDs.12
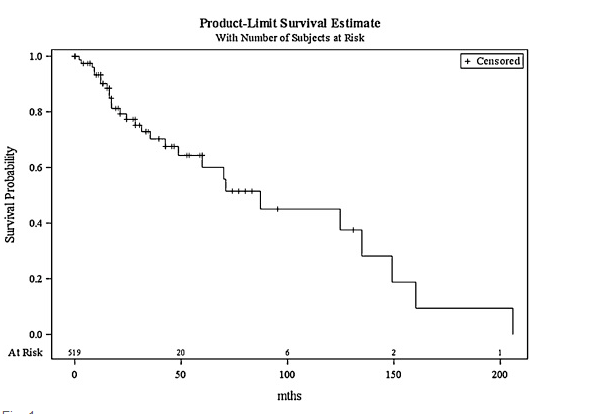
A lack of insight into the progression of PMDs has resulted in a variety of proposed treatments based upon clinician preferences and experience. PMDs are mucosal lesions only, so do not require the aggressive treatment necessary for removal or destruction of invasive cancer. It seems self-evident, therefore, to intervene early to excise dysplastic tissue at this ‘pre-invasive’ stage.
Interventional laser therapies evolved following the demonstrable failure of observational and medical treatments and limitations in conventional surgery to treat oral PMDs.13; 14; 15; 16 ; 17 The use of carbon dioxide (CO2) laser facilitates surgical management, providing precise excision, full histopathological lesion assessment, minimal postoperative morbidity, and coordinated patient follow-up.14; 17 ; 18 Whilst excision is preferred, a limited role for ablation allows a defocused laser beam to destroy small or less dysplastic lesions, particularly at gingival/alveolar sites.16
Previous studies on the treatment of PMDs are limited by small numbers of patients and varying follow-up periods. Detailed, long-term analysis of a large, well-defined PMD patient cohort undergoing coordinated treatment and follow-up was overdue.
In the absence of national PMD disease statistics or a robust evidence base for treatment, the aims of this study were to profile patient demography and clinicopathological data for a patient cohort presenting to a specialist PMD service in the north-east of England, to determine clinical outcomes following interventional CO2 laser surgery and to assess treatment efficacy and its influence on the progress of oral carcinogenesis.
Method
Caldicott approval was obtained from the Newcastle University/Newcastle upon Tyne Hospitals NHS Foundation Trust to facilitate anonymized, retrospective data collection from the medical records, operating logs, and pathology reports of patients with PMDs treated at Newcastle Dental Hospital and the Royal Victoria Infirmary. Inclusion criteria required a new, untreated, single-site PMD, confirmed by incision biopsy. Patients with a previous or multifocal PMD and those with a history of OSCC or head and neck radiotherapy were excluded.
Patient and treatment details
The following demographic and clinicopathological details were collected for patients who had undergone CO2 laser surgery between August 1996 and December 2014: date of treatment, age and sex, clinical appearance and anatomical site of the oral lesion, histopathology diagnoses for incision and post-laser excision biopsies, follow-up data, and any further laser treatment. The clinical outcome was determined at the study census date (31 December 2014), unless malignancy supervened when this became the study exit point.
Histopathology diagnoses
Incision biopsies were performed under the direction of the first author (PJT) and excision specimens were obtained following interventional laser treatment, as detailed previously.medical fibers,14 Laser surgery was performed by the first author (PJT), or by colleagues working under direct supervision; this was done within 6–12 weeks following incision biopsy to avoid disease progression. Formalin-fixed tissue specimens were assessed via standardized histopathology examination by oral pathologists at the Royal Victoria Infirmary working to agreed diagnostic criteria with peer review and consensus grading.4 Using the World Health Organization classification, specimens were graded as mild, moderate, or severe dysplasia, carcinoma in situ, or OSCC. In addition, the presence of hyperkeratosis and lichenoid inflammation (LI) was recorded, as well as diagnoses of PVL and chronic hyperplastic candidosis.
Clinical outcome categories
By reviewing the case records, the patients were assigned to one of the following clinical outcome categories: disease-free (the absence of PMD), further/disease-free (further disease achieving disease-free status following additional intervention), further/persistent (further disease persisting despite intervention), and malignant transformation (if OSCC was confirmed by histopathological examination).
Statistical analyses
Descriptive statistics were used to summarize details of patient demography, clinical features, and pathological diagnoses, together with documentation of treatment interventions, clinical outcome, and follow-up data. Histopathology diagnoses were treated as categorical variables, and testing of agreement between incision biopsy and post-laser excision biopsy diagnoses was performed using weighted kappa statistics; a coefficient of 1 represented perfect agreement.
Clinical outcomes were stratified as disease-free or persistent disease (further PMD or malignant transformation). Success rates and 95% confidence intervals (CI) were calculated. Multivariate logistic regression was used to analyze factors potentially prognostic of a disease-free outcome (age at first treatment, sex, lesion appearance, anatomical site, and histopathology data). Relationships between factors were explored using χ2 tests for categorical variables or logistic regression to check for collinearity. Factors were first explored through univariate analysis and then a multivariate model was built using a stepwise procedure until all variables were significant at the 10% level. Candidate variables for the initial model were those significant at the 20% level on univariate analysis. If collinearity was suspected, only the most significant univariate relationships were included in the model. Malignant transformation was analyzed using univariate Cox regression to investigate clinicopathological variables that might influence time to transformation. All statistical analyses were performed using SAS/STAT 9.3 software (SAS Institute Inc., Cary, NC, USA).
Discussion
Related Articles
- Pulsed Nd:YAG Laser Treatment for Failing Dental Implants Due to Peri-implantitis
- Laser Treatment for Failing Dental Implants
- LOW LEVEL LASER THERAPY
- The clinical observation of semi-conductor laser treatment of hypersensitive dentine
- Interventional laser surgery for oral potentially malignant disorders: a longitudinal patient cohort study
- Er-YAG LASER AND DENTAL CARIES TREATMENT
- Er:YAG laser applications in dentistry
- Topical Gel Application and Low Level Laser Therapy on Related Soft Tissue Traumatic Aphthous Ulcers
- Medical fibers:A New Approach to Relieve Pain in Some Painful Oral Diseases
- Indications and limitations of Er:YAG laser applications in dentistry
